Classification: 8 Tips for Precise Pigeonholing
Medical device manufacturers come across the concept of “classification” in various different contexts: when developing devices and having them authorized, when structuring organizations, and when writing scientific texts.
In this article you will learn:
- Why poor classifications can jeopardize your success
- How you can avoid the typical mistakes
- How to form classes in a way that ensures clarity - the basis for effective and efficient work
1. What classification and classing are
a) Differentiation between classification and classing
The task of classification is to define classes – also called categories – and to assign elements to these classes.
Strictly speaking, classification and classing are different:
- Classification
Forming classes by defining common characteristics. It is the same as creating categories and, therefore, is also called categorization.
- Classing
Classing is the assignment of elements to the previously defined classes based on their features and classification rules.
However, in everyday language, we don’t differentiate between classification and classing and use the term “classification” for both.
b) Examples Medical device manufacturers must assign classes, e. g.:
Medical device manufacturers must constantly:
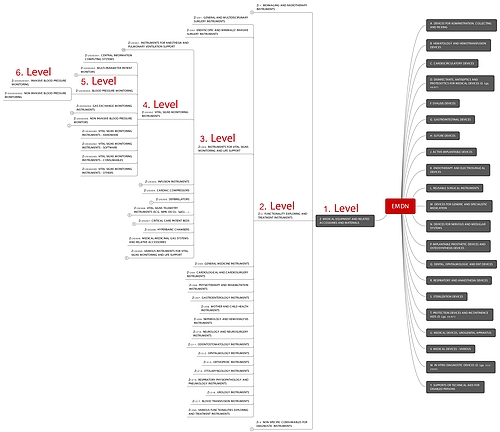
- Classes (types) have to be assigned to medical devices according to the Universal Medical Device Nomenclature System (UMDNS), the European Medical Device Nomenclature (EMDN) or the Global Medical Devices Nomenclature (GMDN) (German).
- The Medical Device Regulation 2017/745 (MDR) and EU Regulation 2017/746 on in vitro diagnostic medical devices (IVDR) require medical devices to be assigned to classes I, IIa, IIb or III, or A, B, C and D, so that the correct conformity assessment procedure can be selected.
- In risk management, manufacturers divide the probabilities and the severities of harm into classes in order to quantify risks.
- In data transmission, semantic standards such as the International Statistical Classification of Diseases and Related Health Problems (ICD-10), the Operation and Procedure Classification System (OPS) and the Anatomical Therapeutic Chemical (ATC) Classification System, are needed to achieve interoperability (German). These standards are hierarchical classification systems, i.e., taxonomies.
2. How the quality of classifications affects you
a) How bad classifications hurt you
Two things can go wrong with classifications:
- You do not create the right classes (classification in the strict sense)
- You assign concepts to the wrong classes (classing)
Both errors can be very damaging for manufacturers, as the following examples show:
Error | Consequence |
Classification according to Annex VIII of the MDR The MDR and MDCG (esp. 2019-11 and 2021-24) establish unclear and sometimes contradictory classification rules for software. | Legal wrangles There have been arguments, all the way through to legal disputes, between manufacturers and authorities/notified bodies. |
EMDN The EMDN is not comprehensive or ambiguous, for example, in the case of non-active accessories for active devices. | No authorization Manufacturers cannot assign their products to a class. The authorization application fails. |
Severity of harm in risk management Manufacturers do not precisely define the rules for the division of severities in risk management. For example, they define serious harm as a “serious impairment of health.” | Delayed submission and authorization The preparation of the risk analysis takes a disproportionately long time because there is no consensus on when harm is considered a “serious impairment of health.” Furthermore, the notified body questions the division of specific risks. The result is a delayed submission. |
Structure of the technical documentation The manufacturer submits the technical documentation electronically. A reader cannot tell from the directory structure (a hierarchical classification) whether the usability test report can be found in the “Usability” directory or in the "Verifications and validations” folder. | Non-conformity The manufacturer's team itself does not know which directories data should be stored in. This leads not just to arguments, it also leads to documents not being submitted or documents being submitted when they are not needed, sometimes even in different versions in different directories. The notified body cannot find the documents and writes a deviation because the very first requirement of Annex II of the MDR/IVDR (“clear, organised, readily searchable and unambiguous manner”) has not been met. |
Structure of the company The organizational structure and workflows do not assign responsibilities and decision-making powers to roles and departments in a way that is clear and comprehensible to everyone. | Non-conformity, loss of customers Different departments bicker about who is responsible for doing something and who is accountable for it. Arguments start. Customers do not know who they should contact. The organization does not adequately meet their needs, so they find another company. |
b) How good classifications benefit you
When you define classes or categories precisely, you show that you have really understood a domain. You create clarity. And clarity creates effectiveness and efficiency.
And with clarity, common problems can be avoided:
- Unnecessary discussions and arguments
- Misunderstandings
- Lack of coordination, e.g., due to different priorities
The division of concepts into classes is like modeling. These models are simplified representations of the world through which you can understand things. For example, models help to identify and eliminate the real causes of problems.
Models also help to formalize knowledge.
3. How to achieve a good classification
Tip 1: map the domain completely
Make sure that you fully map the domain with your classes.
Examples:
- Risk management
- A severity axis that has the death of a patient as the highest class of harm is incomplete if the device could cause the death of more than one patient.
- Organizational structure
- The organigram should include all roles (which are classes with the same activities).
Tip 2: choose the right resolution
Classification, like all modeling, must be fit for purpose.
Examples:
- Risk management
- It is important that a manufacturer of sticking plasters can differentiate between different cases of minor harm for them to be able to make a decision on risk acceptance. Was it just a scratch? Could you see skin irritation? Was medical intervention necessary?
- For the manufacturer of a cardiac catheter, such distinctions should be irrelevant. Instead, it has to differentiate according to whether an error caused surgery to last longer, whether a life-threatening situation resulted, or whether the patient was irreversibly harmed or even died.
- Diseases
- For the distribution of health care funding, it may be enough to divide diseases into a few classes, e.g., “cardiovascular system diseases” or “malignant neoplasms.”
However, for an oncologist, the class “malignant neoplasms” is not very helpful as all their patients fall into this class. They need a much more detailed and nuanced grouping to be able to decide on the right therapy.
Tip 3: pay attention to the integrity of the classification
The classification should maintain its inherent logic, such as the dimension and the system behind the division.
(Counter-)examples
- Risk management: Dimension
- A severity class may be defined as “death” but not as the “possibility of death.” Because “possibility” implies a probability. And that is another dimension.
- Risk management: Scale
- Manufacturers often define probability classes on a logarithmic scale. They should stick with this and not change to a linear scale or combine different orders of magnitude in the same class.
Tip 4: avoid overlapping classes
To allow a clear assignment of concepts to classes, the classification rules must ensure unambiguous and reproducible assignment. This is not a minor issue, since the world cannot always be represented as classes or hierarchical classes, i.e., as a tree.
Examples
- Types of medical device
- Does an implantable defibrillator belong to the class “defibrillators” or does it belong to the “active implantable medical device” class? Such a decision cannot be made solely on the basis of class names. Additional classification rules are needed.
- Diseases
- The ICD-10 catalog differentiates between the classes “neoplasms” and “disease of the digestive system.” But which class does colorectal cancer fall into? Additional rules are again needed to ensure clarity.
Tip 5: choose intensional instead of extensional groupings
There are different approaches for determining which class certain concepts should be assigned to. We differentiate between:
- Intensional grouping
- The division is defined using characteristics or rules.
- Extensional grouping
- The division is “defined” using examples and/or by listing.
Examples
- Risk classes according to the MDR and FDA
- Annex VIII of the MDR establishes rules for classifying medical devices (intensional grouping). In contrast, the FDA lists the device types (device codes) for each class.
- Organization
- An organizational structure can be defined by an organigram showing which departments make up the individual areas (extensional). Or there can be a rule that allows this assignment.
Intensional grouping has the advantage that it does not have to be adapted, i.e., expanded, in the event of new elements, as is the case with extensional groupings.
However, intensional grouping has the disadvantage that divisions occur because rules are missing or are not suitable for the new element.
Tip 6: define precise and unarguable classification characteristics
The rules and thus classification characteristics must be unambiguous and unarguable. Therefore, we recommend combinations of binary criteria for which an unarguable decision can be made.
Examples
- Risk management: Negative example
- If manufacturers define severity classes with adjectives such as "severe,” “serious” or “catastrophic,” there will be unnecessary arguments.
- Risk management: Positive example
- More helpful is defining a severity class, such as “life-threatening harm” (this is precisely defined in intensive care medicine), “medical intervention necessary” (is always the case if healing otherwise takes longer) or “irreversible damage with at least 20% disability” (pension schemes define the degrees of disability precisely).
Tip 7: make sure your categories are comprehensible
The classification rules must be understood by the people who are responsible for the classification (more precisely, the classing). Therefore, unclear terms and examples must be avoided.
Tip 8: pay attention to manageability
Finally, you must ensure the “usability” of your classification rules. In hierarchical classification systems (taxonomies), for example, this means:
- The tree must not be too wide, otherwise it becomes difficult to find the right class. A good rule of thumb is 7 +/- 2 elements per level.
- The tree must not be too deep, because otherwise too many decisions are needed to correctly classify (class) the element. The depth correlates with the width, at least in balanced trees (e.g., AVL trees), depending on the number of elements.
- The granularity must not be too high, otherwise division becomes more difficult. The granularity depends on the purpose of the classification (see Tip 2).
- The class names should enable a quick initial assignment. Precise assignment is then regulated by the classification rules.
4. Conclusion
People say a lot about their thinking through the way they represent the world, how they divide it into categories and classes. Even the directory structure on a drive or the section structure of a document reveals a lot about the owner or author.
Precise classification creates precise models. They, in turn, provide clarity, without which demanding activities such as device development or managing an organization successfully become almost impossible.
Therefore, it is worth taking the time to create a precise classification.
The Johner Institute helps medical device manufacturers and their service providers gain clarity: from the qualification and classification of their devices, to the structure of their technical documentation, through to successful authorization (German) and post-market surveillance.